This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
For the Patient
The intestinal microbiota participates in the processes related to digestion and absorption of nutrients, and the bacteria that are part of the intestinal microbiota perform various functions, their correct quantitative and qualitative structure (referred to as the eubiosis state) supports the homeostasis of the whole organism, shaping immunity, metabolism and synthesis of numerous chemical compounds such as serotonin, neurotransmitter precursors and compounds that seal the gut-blood barrier.
The total mass of bacteria in the gut was calculated to be about 2 kg and they belong to four types: Firmicutes, Bacteroidetes, Proteobacteria and Actinobacteria. The number of bacteria in a gram of colon content is up to 1012 (trillion!) – 1014 (one hundred trillion!) cells, and the total number of species is reaching 800–1000.

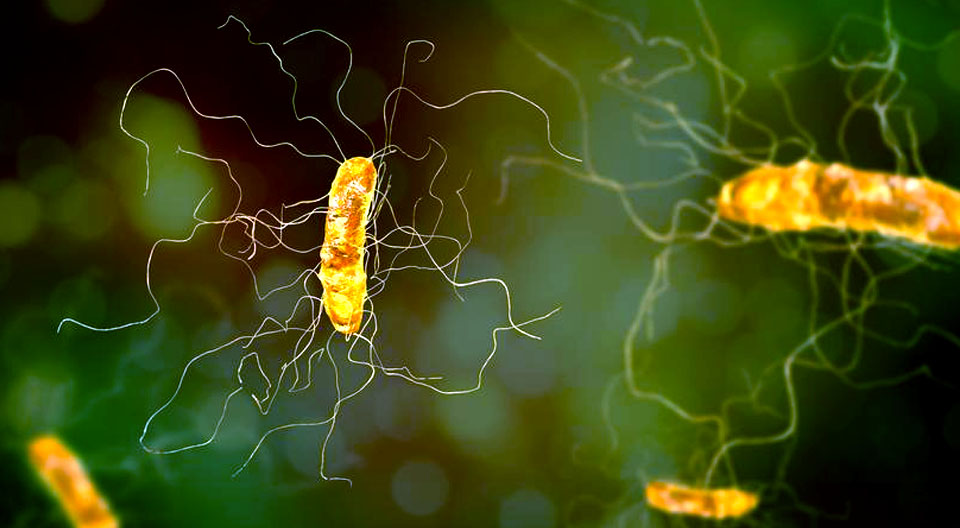
Knowledge of the functions and pathologies resulting from the different composition of the gut microbiota has led to the concept of transferring the composition of the gut from healthy to sick people. Fecal microbiota transplantation was already used in an ancient China, and was reactivated in the 1950s. The advancement of technology, especially describing and discovering the functions of intestinal microorganisms, mainly with the use of culture-independent diagnostic methods (including next-generation sequencing) has led to rapid advances in understanding the tasks of the gut microbiota. It has been discovered that many diseases, pathological conditions and states rest on the intestinal microbiota.
How does the gut microbiota work in Clostridioides difficile infections?
Although the mechanism has not yet been fully established, it is believed that FMT works by the patient’s gut microbiome diversity restoration with typical microorganisms found in the gastrointestinal tract of healthy people. They competitively displace C. difficile from the gastrointestinal tract (by “taking space”, intestinal niches and food).
C. difficile competes with many different species of bacteria in a healthy gut microbiota. However, treatment with antibiotics disrupts this ecosystem, killing bacteria that have a protective effect and the “colonization resistance” is being lost. C. difficile creates antibiotic-resistant spores and, as an overwhelmingly out-competing pathogen, nests in the gut and produces toxins that cause the typical symptoms of infection – severe diarrhea, abdominal pain and fever. Thanks to the transfer of bacteria from the stool of a healthy donor, C. difficile is defeated again. While FMT is an attractive tool for the treatment of C. difficile infections, there are also some risks. Stool is a complex, live mix of bacteria and other organisms. Although stool donors are examined very carefully for known infectious agents, there is always a risk that the tests may fail to detect a certain pathogen or that another pathogen that has not been tested may appear. There are also risks associated with the method of administering FMT – by colonoscopy, gastroscopy, rectal enema, nasogastric or nasojejunal tube. FMT capsules seem to be the safest option. In view of the above, it is important to manufacture the preparation according to a strict regime and the highest standards. The Human Biome was created with the idea of using even safer solutions than the global recommendations say. We care about safety and quality.

How to prepare for FMT procedure?
Attending physician will provide you with all the necessary information about the treatment with FMT. Most often, if your condition allows it, before performing the FMT procedure, a minimum of four days of vancomycin treatment is used (official guidelines say 10-14 days), the antibiotic is discontinued and, regardless of the route of administration, the intestine is cleansed with laxatives, most often osmotically active (macrogols as before a colonoscopy), in order to reduce the bacterial load of C. difficile in the intestines and the gut microbiota preparation is applied into the patients intestines, depending on the method and route of administration. The treatment does not require any special preparation other than that described above. After the procedure, you can return to a normal, healthy diet and avoid factors that may lead to recurrence of the infection (e.g. antibiotics treatment, contaminated surfaces).

How to prevent recurrence of C. difficile infection after FMT treatment?
If you have recently undergone a fecal microbiota transplantation due to C. difficile infection, your healthcare professional has informed you about possible side effects such as nausea, bloating and mild abdominal cramps, cramps, short-term diarrhea. If you have noticed persistent, unusual changes in your health, or experience unusual, negative symptoms after FMT, notify your doctor immediately.
To minimize the risk of reinfection with C. difficile:
-
Disinfect your home with a good disinfectant as C. difficile creates spores that are resistant to common household cleaners. We recommend using disinfectants with a C. difficile sporicidal label, such as household bleach or strong chlorine solution. Be sure to thoroughly scrub touch surfaces, including toilets, faucets, and showers.
-
Protect yourself while cleaning. Wear at least disposable gloves and wash your hands immediately with soap and water after removing them.
-
Contact your doctor. Make sure your GP knows you have undergone a fecal microbiota transplantation for the treatment of C. difficile so that they can consider this history when making decisions about your care. Taking antibiotics after FMT increases your risk of reinfection with C. difficile, but if you get an infection that requires antibiotics, you must take them anyway. Then talk to your doctor about medication options with a lower risk of generating C. difficile infections.

Who can receive a fecal microbiota transplantion?
A standard indication for treatment with FMT, not requiring the approval of the bioethical commission, recognized by global, including Polish, microbiological and infectious disease societies is recurrent and/or severe C. difficile infection. European, American and Polish guidelines collectively say that the FMT procedure can be performed without additional administrative procedures in the following indications:
- a relapse of C. difficile requiring hospitalization,
- at least two previous episodes of C. difficile infection, which did not necessarily require hospitalization,
- moderate C. difficile infection that has not responded to a minimum of one week of vancomycin or fidaxomicin treatment,
- severe C. difficile infection or pseudomembranous colitis that does not respond to a minimum of two days of treatment with vancomycin or fidaxomicin.

How to find a medical facility that provides FMT treatment?
Each public facility (hospital, medical center, clinic) can perform FMT as part of the treatment of C. difficile infections by settling the procedure with the National Health Fund. However, not everyone is aware of that. If you have problems in your facility or you want to find out where FMT treatment is already working well – call us or write to us and provide your place of residence. We will state the nearest facility that performs fecal microbiota transplantation procedures using Human Biome preparations in Clostridioides difficile infections and experimental indications as part of clinical trials or medical experiments. We also cooperate with many private centers throughout Poland, including the recommended Microbiota Centers Microbiota Centers.
Frequently asked questions
Can the Human Biome Institute provide material for self-performing FMT at home?
No. To ensure the safe and appropriate use of gut microbiota preparations and to ensure compliance with the recommendations of global scientific societies, we provide material only to medical units for administration in hospital or outpatient settings. The patient cannot directly order preparations for FMT. However, it is possible if the doctor deems it justified to deliver the preparations to the patient’s home address, but only the doctor decides about it after contact with the patient and his prior preparation and training on how to use our preparations. To find a physician or hospital near you who already performs FMT, call us or write to us. We not only cooperate with state hospitals, but also, we help new hospitals, including private ones, in providing their patients with FMT, as well as outpatient facilities and medical practices. Every gastroenterologist, infectious disease physician, and internist should be able to provide FMT, considering our activities and the widely available FMT program for the treatment of C. difficile. We also provide material for FMT in other clinical indications, as part of therapeutic experiments, research and clinical trials.
Can Human Biome Institute material be used in the treatment of Crohn’s disease, Ulcerative Colitis, Irritable Bowel Syndrome or other diseases?
Currently, only as part of a clinical trial or therapeutic or research experiment. According to the principles of medical art, medical treatments can be performed widely and openly in scientifically documented indications, when given therapies are included in the treatment guidelines for a given disease.
Currently, only in the case of C. difficile infections, the use of FMT without additional approvals is possible. For any other clinical indication, the consent of the Ethics Committee is required.
How much will the procedure with the use of Human Biome Institute preparations cost me?
If you are treated in a state-run institution, the procedure will not cost you anything. The hospital, as part of settlements with the National Health Fund in Poland (NFZ), may purchase any therapy it deems appropriate. They should, if necessary, also obtain FMT for the treatment of patients with appropriate indications. The NFZ reimburses the hospital for the treatment of the patient. The cost of the FMT preparation is about half the cost of treating C. difficile infections, so it is a cost-effective treatment for the hospital.
If you are treated in a private facility, you will bear the cost of the preparation, equipment and staff work – these prices depend directly on the treatment facilities. Certainly, the implementation of the FMT provided by the Human Biome Institute will pay off. Due to the fact that we operate according to unified standards and we do it on a large scale, our price is competitive and much lower than the production of individual preparations in the standard provided by HBI.
Where does the graft material come from? What tests and procedures do donors undergo?
Our fecal microbiota preparations are sourced from healthy donors who have undergone a range of screening tests, including a 100+ item health questionnaire and over 70 blood and stool tests. Our donors are constantly monitored and re-checked at regular intervals. The donor is always examined at least twice before his material is released for treatment. Additionally, each material is tested separately on a daily and 5-days period. We meet the standards set by international scientific societies, but also much more. We probably have the most rigorous donor screening panel in the world. All this to make the material as safe and effective at the same time as possible.
What was the inspiration for creating such a project as the Human Biome Institute?
We started our adventure with the Human Biome Institute as it was a natural extension of our research on the gut microbiota. Having performed FMT treatments for many years and examining them both in clinical and purely scientific terms, we have seen the potential, effectiveness and safety of FMT treatment. The needs of patients, especially patients with impaired immunity, prompted us to create a unit that, with the maximum safety of preparations, will prepare them as best as possible, in order to maintain a healthy repertoire of microorganisms in the therapeutic suspension, and at the same time will continue to conduct scientific research. Everything for patients, their welfare and health. Everything for science that should be constantly updated.

