For Physician and Medical Facility
For Physician
The art (but also satisfaction) of medicine is based on continuous education, improvement and treatment of patients in accordance with the latest medical knowledge. According to this knowledge, fecal microbiota transplantation (FMT) is the most effective therapy in the treatment of recurrent and severe Clostridioides (formerly Clostridium) difficile (CDI) infections, and probably also a number of other diseases, conditions and abnormal conditions (research and studies clinical trials are ongoing).
The strength of the recommendations in both European and American recommendations regarding the treatment of CDI with FMT has the power of A I, which means that there is no better therapy than gut microbiota transplantation in this indication. The effectiveness of a single treatment is in most studies and meta-analyzes over 90%. We rarely see such high cure rates in medicine. HBI preparations are in line with the world’s best statistics!
Fecal Microbiota Transplantation is:
- scientifically tested, safe and reliable (has extremely high cure rates),
- adjusted to the diseases it cures – “tailored” for the patient,
- the latest solution tailored to the problems of today’s medicine – antibiotic resistance, aging populations, the pharmaceutical industry’s failure to keep up with the production of new drugs,
- medical innovation of the last decade and
- the basis for the creation of drugs derived from microbiota (the research part of the R&D Human Biome works on this!)

At the Human Biome, we want to support Polish doctors in the use of fecal microbiota transplantation by providing a safe, validated preparation and knowledge of how to use it. We want to help develop treatment in Poland based on the latest – world discoveries. In addition, and in fact above all, we conduct continuous scientific research, using methodology and technologies from the highest world-class.
Without the active involvement of doctors, even the greatest medical discovery will remain only a discovery and not a real help for the patient. Below you will find scientific organization guidelines for the place of FMT in the treatment of C. difficile infections.
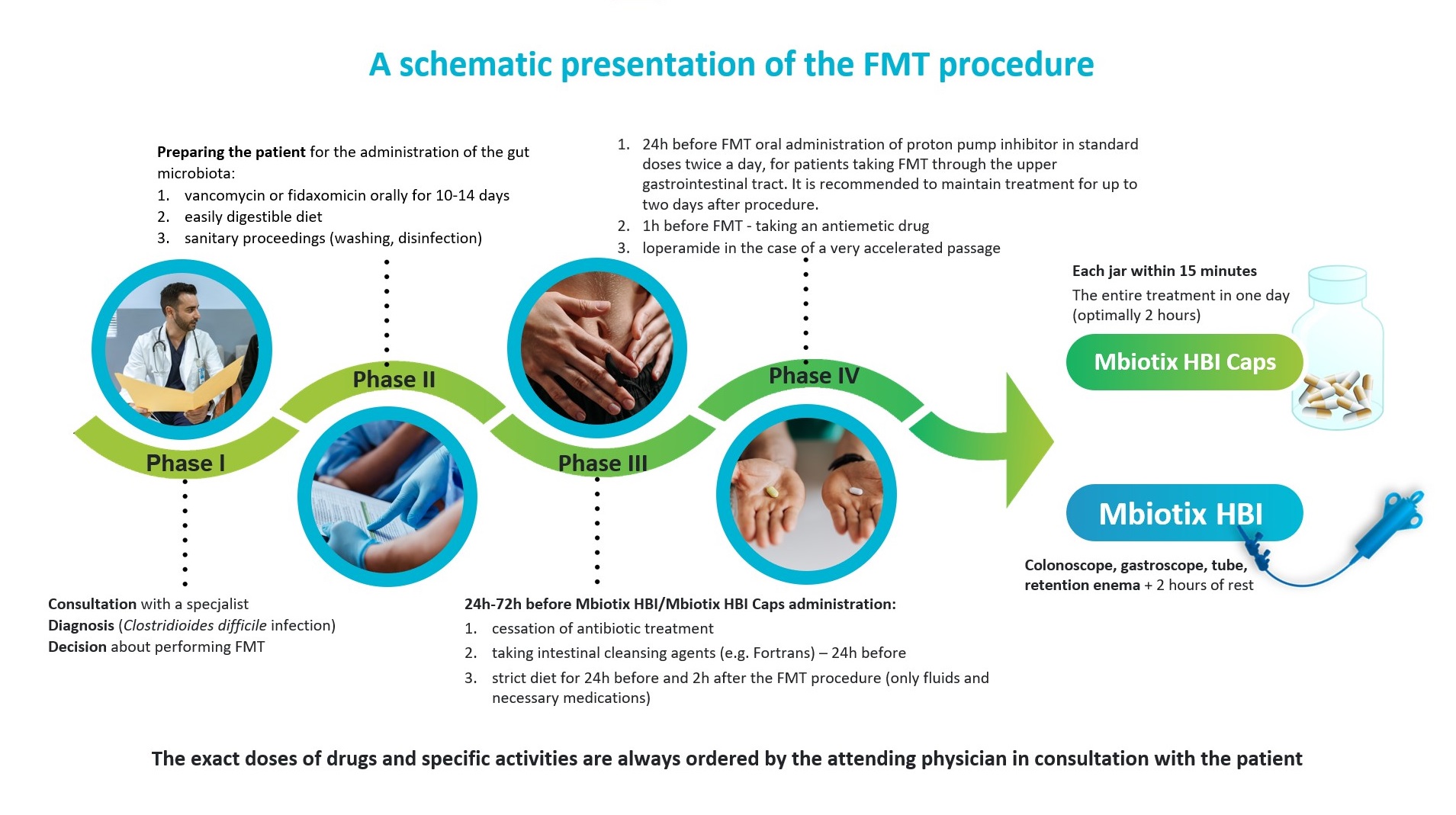
FMT IN THE TREATMENT OF C. DIFFICILE INFECTIONS – STEP BY STEP
-
1. According to the recommendations in recurrent C. difficile infections, it is recommended to administer vancomycin in doses of at least 4 × 125 mg before the planned FMT, for a period of at least 10-14 days (literature data indicate that vancomycin treatment for 4 days may already be effective) with subsequent administration of microbiota (however, this is still knowledge not included in the official guidelines) or fidaxomicin 2 × 200 mg for 10 days.
-
2. A few days before FMT, the patient should be on an easily digestible diet.
-
3. The administration of antibiotics to the patient should be stopped for a minimum of 24 hours, but not longer than 72 hours before the administration of MBiotix® HBI and/or MBiotix® HBI Caps. Taking antibiotics at the same time may reduce the effectiveness of the therapy.
-
4. The day before FMT, the patient should be given intestinal cleansing agents (as for colonoscopy). The administration of cleansing agents is important and does not depend on the method of FMT administration – it is always recommended. Increases the chances of successful implantation of the donor’s gut microbiota. From that moment (cleansing the intestine) until two hours after the procedure, the patient should be on a strict diet (can take fluids and necessary medications).
-
5. 24 hours before MBiotix® HBI administration (when FMT is administered via the upper gastrointestinal tract – by intragastric, intraduodenal, enteral, gastroscopy or PEG tube infusion, and using MBiotix® HBI Caps capsules), it is recommended to start orally administer a proton pump inhibitor (IPP) in standard doses twice daily. It is recommended to maintain treatment for two days after procedure.
-
6. One hour before performing FMT with capsules, gastric tube, intraduodenal / enteric tube and possibly gastroscopy – it is recommended to administer an antiemetic to the patient (8 mg ondansetron orally or intravenously is recommended).
-
7. On the day of FMT administration (infusion into the gastric, intraduodenal, enteral or gastroscopy tube), in the case of accelerated intestinal transit, it is recommended to administer loperamide (dose just before taking FMT 2mg-4mg and one hour (and possibly 6h) after taking FMT 1-2 mg) to slow down gastrointestinal transit. Loperamide is not recommended in patients at high risk of developing toxic colonic distension or gastrointestinal paralytic obstruction and in the case of administration in capsules, unless gastrointestinal transit is evidently accelerated.
-
8. According to the literature, the most effective form of FMT administration is colonoscopy and capsules (both methods are more than 90% effective in the treatment of CDI). However, depending on the situation, it is the doctor and the patient who choose the form of administration of the material.
-
9. After FMT performed by the upper gastrointestinal tract, the patient should stay in a sitting / semi-sitting position for at least two hours.
-
10. After colonoscopy or rectal enema, the patient should keep the material for at least 30 minutes (recommended 2 hours). The patient may lie in the Trendelenburg position and turn from side to side.

For Medical Facility
Polish hospitals are more and more modern and better managed.
Professionalism, economic ability, soft skills, vision and openness to innovation – these are the qualities of hospital managers in Poland. We are very happy to notice that apart from taking care of the financial results, managers of institutions efficiently communicate with the staff, take care of the image of the centers, remain open to new treatment technologies, provide the staff with the possibility of development and – very importantly – choose therapies that really help patients.
- 6 000 euro is the minimum cost of a single hospitalization of a patient with Clostridioides difficile,
- 50% (minimum) – 50% (minimum) – hospitalization costs increase, which generates each relapse of infection,
- 56 000 euro – total annual cost of recurrent Clostridioides difficile infection when FMT is not used,
- 32 000 euro – total annual cost of recurrent Clostridioides difficile infection if FMT was used
- 42% – this is the reduction of hospitalization costs after using FMT (from 56,000 to 32,000 euros per year). It just pays off!

How to get fecal microbiota material for your facility?
If a patient in your facility requires the use of a fecal microbiota due to infection with Clostridioides difficile or for any other indication, if you have obtained approval from the ethics committee, please contact us. Write us a message in the contact tab, call us or write an e-mail. We will help you pass the administrative parts as quickly as possible to deliver the material for the patient’s treatment as soon as possible.
